Innovation drives everything
Promoting progress in clinical practice. Carrying out clinical research.
Realising research cooperation with external partners. Investing in multi-centre studies. Bundling new insights and knowledge. Being a forum for experts and the voice of our field of expertise. Innovation really drives things forward at Dr. Falk Pharma. Today, as it always has.
Research and Development
The core here is the many years of experience in the areas of pharmaceutical, preclinical and clinical development. The teams of Dr. Falk Pharma implement the development projects in close cooperation with contract research organisations.
In galenics, Dr. Falk Pharma has developed outstanding expertise in topically - i.e. locally - effective drugs for the intestinal tract and the oesophagus.
This has led and will lead to a whole host of patents and clinical approvals.
Multi-centre studies play an essential role in the approval of new drugs.
The teams of Dr. Falk Pharma are very experienced in organising large multi-centre studies and have been able to establish an international network of clinical investigators over many years. This means that even extensive studies can be carried out quickly and successfully.
Investment in three dimensions
The aim is to further expand the range of therapeutic options in the field of digestive and metabolic medicine.
The further development of existing forms of therapy:
Areas in which the company is a leader and is successful.
For example with new galenics for existing products.
The launch of new forms of therapy:
Currently, Dr. Falk Pharma is the first to offer a product for the clinical condition of Eosinophilic Oesophagitis (EoE): a chronic inflammatory, immune-mediated disease of the oesophagus that leads to swallowing difficulties.
The development of new indication areas:
Dr. Falk Pharma is therefore investing significantly in studies researching into new active agents for clinical indications which need far more effective therapies: such as primary sclerosing cholangitis (PSC), non-alcoholic steatohepatitis (NASH) and coeliac disease and gastroparesis.
Partnering – stronger together
We continuously strive for transforming ideas and current advances in medical treatment. Dr. Falk Pharma cooperates with reliable partners in order to develop and implement high potential innovative treatment concepts. These can arise from any pharmacological concept: from “small molecules” to biologics. Within these projects, a wide variety of developmental steps are considered, which can have their origin in academic research, in clinical use, in start-ups or even established companies.
We look forward to making new contacts and establishing new partners, both nationally and internationally, in the fields of digestive and metabolic medicine. Feel free to contact us directly.
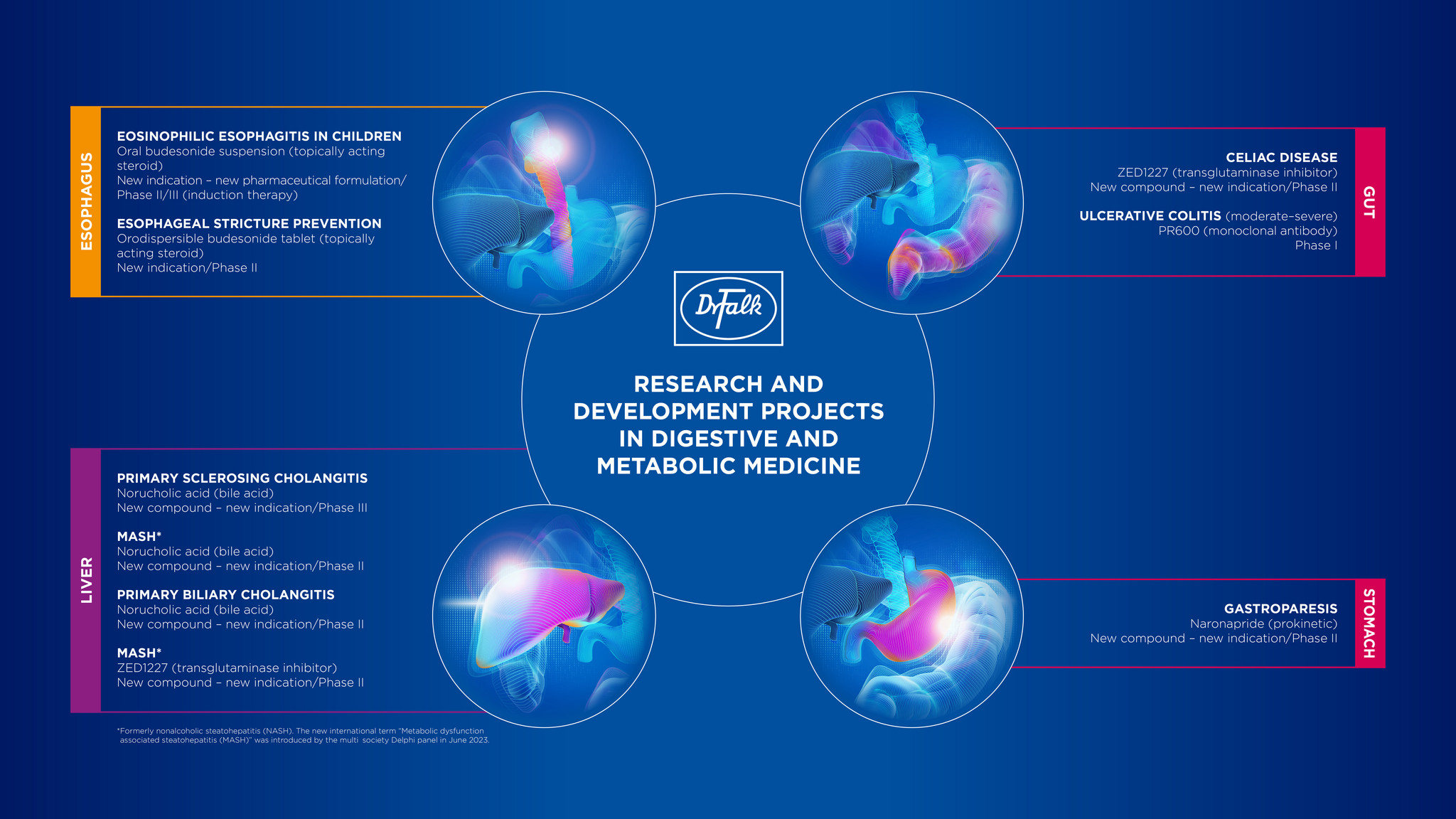
Current research and development activities
The focus of our current development projects covers both new drugs for inflammatory bowel disease, other bowel disorders, hepatic, biliary, and oesophageal disorders, as well as the continuing development of previously-approved drugs - for example through the use of improved formulations. We are also investigating new therapeutic indications for currently-approved drugs.
The following research projects are the particular focus of our present work:
- Ulcerative colitis: PR600 (mAb)
- Ulcerative colitis: Budesonide in an extended-release formulation
For more detailed information on the conditions of Crohn’s disease and ulcerative colitis, please visit the indications page.
- Coeliac disease: ZED1227
- Gastroparesis: Naronapride
- Primary sclerosing cholangitis: Norucholic acid
- Primary biliary cholangitis: RhuDex & Norucholic acid
For more information on primary biliary cholangitis, click here.
- Nonalcoholic steatohepatitis: Norucholic acid & ZED1227
- Pediatric eosinophilic oesophagitis (under age 18): Budesonide suspension
- Oesophageal stricture prevention: Orodispersible budesonide tablets with effervescent properties
For more details on eosinophilic oesophagitis, click here.
An overview of our entire research and development program can be found at EudraCT or in the graphic below.